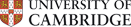The Carroll lab is interested in understanding how Estrogen Receptor (ER) causes gene transcription and how this contributes to breast cancer progression. We are also interested in understanding how breast cancer therapies work and what happens if they fail. We explore this using molecular, genomic and proteomic approaches, with the goal of identifying the critical determinants of tumour progression so that we can develop novel therapies that specifically target them.
The global questions in the lab are: 1. What allows Estrogen Receptor (ER) to switch genes on in breast cancer cells? 2. how do drugs like tamoxifen work to block ER function and what happens when these drugs fail? 3. Can we use this information to develop better therapies for breast cancer treatment?
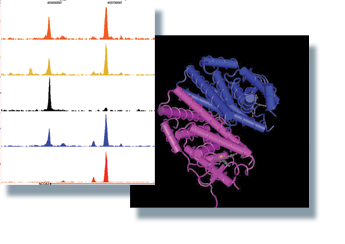
Our work has focused on defining and characterising novel Estrogen Receptor (ER) associated proteins in breast cancer cells and the genomic cis-regulatory elements. This includes factors that mediate ER-chromatin interactions, such as TLE1 (Holmes et al, 2011) and FOXA1 (Hurtado et al 2010). We have found a role for RARα as an ER interacting factor, co-operating with ER in the presence of estrogen, but antagonising ER function in the presence of retinoids (Ross-Innes et al 2010). Other factors we have identified are utilised by tamoxifen-ER for active repression of transcription, including PAX2 (Hurtado et al, 2008).
Our lab pursues a number of technical and methodological projects to circumvent limitations of current systems. We apply these to better define transcription factor biology and endocrine response in breast cancer. As an example, we have developed ChIP-seq approaches for transcription factor (ER) mapping in primary tumours and core biopsies. By establishing and utilising this approach, we can determine differential ER regulatory regions in primary tumours, metastases and normal tissue. This permits genomic understanding of ER binding dynamics in breast cancer progression and drug resistance.
Our current interests include the characterisation of novel ER associated proteins using newly developed proteomic approaches. We are also interested in the cross-talk between ER and AR in breast cancer. We are particularly interested in the role that FOXA1 plays in mediating ER-chromatin interactions and we are exploring mechanisms that dictate FOXA1 binding dynamics. We are also developing therapeutic inhibitors against some of the factors we have characterised, with the goal of discovering treatments that will be effective in breast cancers that have acquired resistance to traditional therapies.
08 Apr 2024
17 Mar 2024
18 Jan 2024
15 Dec 2023
20 Oct 2023
10 Apr 2023
10 Nov 2022
30 Sep 2022
15 Sep 2022
20 Aug 2022
Back to top